Genomic rearrangements are vital in genetic diversity, facilitated by enzymes involved in DNA repair and genetic material movement, such as transposases and recombinases. These enzymes are utilized by mobile genetic elements (MGEs) to mobilize DNA, ranging from site-specific to semi-random insertions and deletions. Insertion sequences (IS), found extensively in bacteria and archaea, typically employ transposases that recognize terminal inverted repeats. In contrast, the IS110 family utilizes a distinct DEDD catalytic motif for a cut-and-paste mechanism, lacking terminal inverted repeats. This family targets specific genomic sequences, often integrating into repetitive elements within genomes.
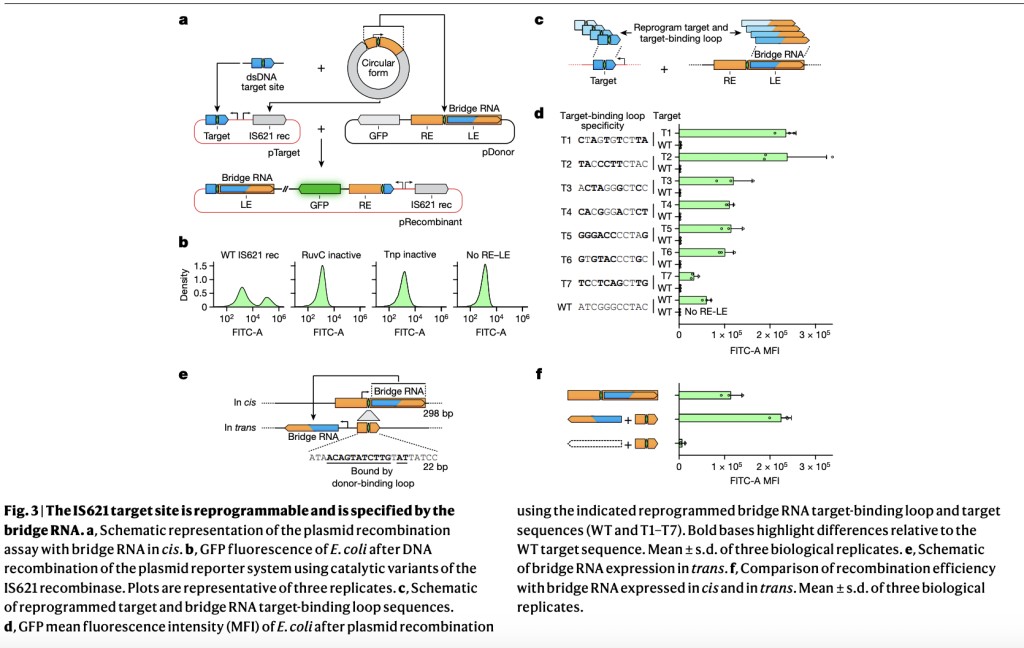
Researchers from several institutions, including the Arc Institute and UC Berkeley, found that IS110 insertion sequences express a structured non-coding RNA (ncRNA) interacting with their recombinase. This “bridge†RNA has two loops that specifically bind to both the target DNA and the donor DNA of the IS110 element. These loops can be reprogrammed independently to facilitate sequence-specific recombination, allowing DNA segment insertion, excision, or inversion. This system offers a new, modular approach to DNA rearrangement, expanding capabilities beyond traditional methods like CRISPR.
The IS110 family, including IS621, utilizes specialized recombinases with DEDD RuvC-like and serine-rich domains for excising and integrating into bacterial genomes. The key to this process is that IS621 recombinase binds to a specific ncRNA derived from its ends. With a structured sequence of stem and internal loops, this ncRNA bridges the recombinase and DNA during integration. The conserved nature of ncRNA across IS110 elements and its critical role in recombination suggest the potential for programmable DNA targeting. This understanding enhances our grasp of bacterial genome dynamics and hints at applications in precise genetic modifications.
The study developed a vast sequence database from sources like NCBI and MGnify, containing metagenomes, MAGs, and viral genomes. IS110 protein sequences were annotated, clustered, and analyzed for conserved residues and phylogeny. The research included identifying IS110 element boundaries through comparative genomics and iterative BLAST searches. Bridge RNA structures flanking recombinase genes were predicted and analyzed for nucleotide covariation. Techniques such as small RNA-seq, in vitro transcription, and plasmid recombination assays in E. coli were employed. Key tools included mmseqs2 for clustering, iqtree2 for phylogenetics, and MST for binding affinity measurements.
The experiments with the IS621 recombination system in *E. coli* demonstrated its high specificity and programmability. Researchers achieved targeted recombination with significant precision by using customized bridge RNAs that precisely match target DNA sequences. The system strongly preferred exact matches and mismatch tolerance was low, indicating high fidelity. Recombination efficiencies were notably improved when bridge RNAs were expressed separately (in trans). This ability to reprogram IS621’s bridge RNA for precise DNA targeting and insertion highlights its potential for accurate genomic engineering with minimal off-target effects, making it valuable for therapeutic and biotechnological applications.
In conclusion, the discovery of bridge RNA represents a novel paradigm in RNA-guided genetic manipulation, distinct from known systems like tRNA, CRISPR RNAs, and snoRNAs. Unlike single-strand RNA guides, bridge RNAs are bispecific molecules that orchestrate the precise alignment of donor and target DNA sequences for efficient recombination. This compact RNA (around 150–250 nucleotides) partners with a recombinase protein (around 300–460 amino acids), utilizing internal binding loops akin to tRNA or snoRNA structures. This mechanism allows IS110 elements, widely distributed in prokaryotes, to execute diverse DNA rearrangements with minimal reliance on protein-DNA interactions. Structural studies reveal a modular RNA-protein complex capable of catalyzing intricate DNA manipulations like insertion, excision, and inversion, positioning bridge RNAs as powerful tools for genome engineering beyond conventional RNA-guided systems.
Check out the Paper 1 and Paper 2. All credit for this research goes to the researchers of this project. Also, don’t forget to follow us on Twitter.Â
Join our Telegram Channel and LinkedIn Group.
If you like our work, you will love our newsletter..
Don’t Forget to join our 45k+ ML SubReddit
Create, edit, and augment tabular data with the first compound AI system, Gretel Navigator, now generally available! [Advertisement]
The post What if We could Universally Edit Any Two Pieces of DNA? Meet ‘Bridge Editing’ and ‘Bridge RNA’: A Modular Approach to RNA-Guided Genetic Rearrangements in Bacteria appeared first on MarkTechPost.
Source: Read MoreÂ