The integration of AI in clinical pathology faces challenges due to data constraints and concerns over model transparency and interoperability. AI and ML algorithms have shown significant advancements in tasks such as cell segmentation, image classification, and prognosis prediction in digital pathology. Despite outperforming pathologists in specific functions like predicting colorectal carcinoma microsatellite instability, regulatory hurdle,s and ethical considerations hinder their widespread clinical adoption. Collaborative approaches where AI assists pathologists have proven beneficial, enhancing diagnostic accuracy and efficiency. However, current AI models often need more ability to provide clear explanations at the single-cell level, limiting their broader applicability in pathology practice.
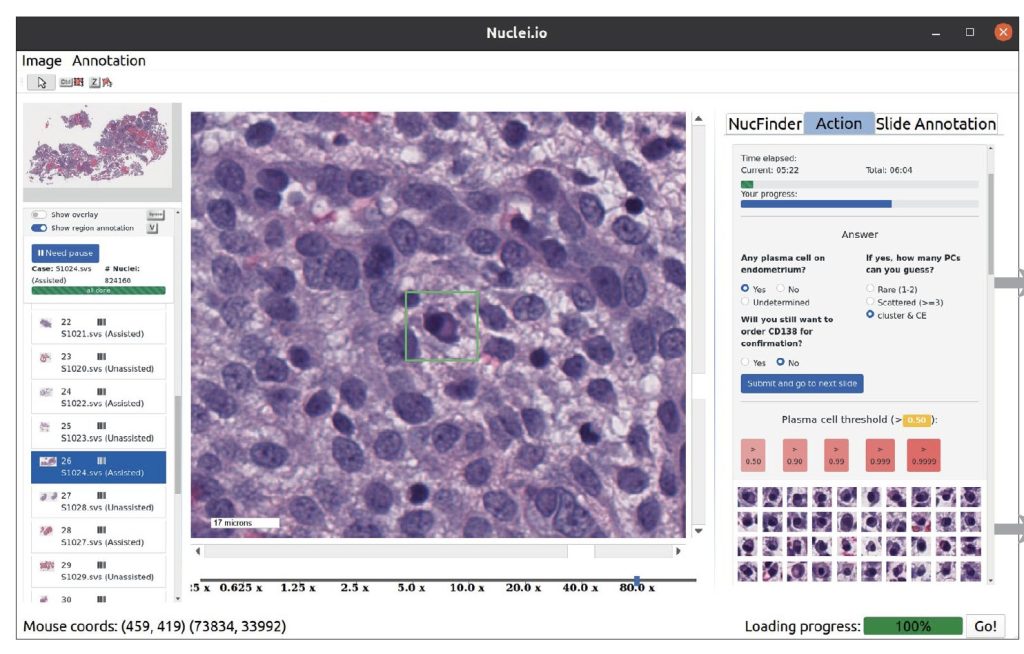
Researchers from Stanford University developed nuclei.io, a digital pathology framework integrating active learning and real-time human-in-the-loop feedback. This framework enhances the creation of datasets and models for various pathology applications, focusing on interpretable features from standard H&E staining. Two studies validated nuclei.io’s effectiveness: one identified plasma cells in endometrial biopsies, improving sensitivity and reducing diagnosis time; the other detected colorectal cancer metastasis in lymph nodes, enhancing sensitivity for isolated tumor cells. These studies underscored the framework’s capability to boost diagnostic accuracy and efficiency through collaborative interaction between pathologists and AI, demonstrating its potential across diverse clinical tasks.
Two studies using the nuclei.io digital pathology framework were conducted to evaluate its effectiveness in assisting pathologists across different surgical pathology tasks. The research employed digitally scanned cases previously diagnosed at Stanford Medicine, comparing pathologists’ evaluations with and without ML support. Each pathologist examined the same cases twice, alternating between assisted and unassisted modes to minimize bias. The first study focused on identifying plasma cells in endometrial biopsies using an expert-trained ML model, significantly improving accuracy and reducing uncertainty and the need for additional tests. The studies demonstrated that ML assistance enhances pathologists’ diagnostic accuracy and efficiency in digital pathology tasks.
The study utilized nuclei.io, a bespoke desktop software, for annotating data, visualizing images, and facilitating pathologist-AI collaborations. For prostate cancer (PC) identification, images were displayed on a 32-inch monitor, while a 24-inch monitor was used for colorectal cancer (CRC) lymph node metastasis detection. The software, operating on Ubuntu 20.04 LTS, was developed in Python 3.9.7 with PySide6 for the GUI, integrating numerous packages like numpy, matplotlib, and TensorFlow for nuclei segmentation and feature extraction. Active learning, employing XGBoost for classification, prioritized uncertain data points for pathologist annotation, enhancing efficiency over random sampling. Comparative studies with QuPath demonstrated nuclei.io’s superior performance in model development, achieving higher F1 scores for tumor cell and PC classifications within shorter annotation times.
The study explores how ML models enhance pathologists’ efficiency and accuracy in diagnosing PC and identifying CRC lymph node (LN) metastasis. ML significantly reduced pathologists’ time per case by 62.05% on average for PC diagnosis, with PC-positive cases showing a 65.94% time improvement. Pathologist experience influenced efficiency gains, with fellows benefiting the most (65.11% reduction). For CRC LN metastasis, individualized ML models improved sensitivity and F1 score, particularly benefiting attending pathologists in detecting micro-metastases. Challenges included varied sensitivity outcomes for residents with ML assistance, emphasizing the need for tailored training and real-time adjustments to maintain accuracy and trust. Overall, integrating ML in pathology promises faster, more accurate diagnoses, though further research is needed to refine models and ensure their seamless integration across diverse clinical settings.
In conclusion, Integrating AI/ML in digital pathology presents significant potential to streamline tedious tasks. However, its adoption must be improved in medical settings due to challenges such as insufficient labeled data, variability in slide characteristics, and the need for transparent and interpretable models. The study introduces nuclei.io, a Python-based software enabling real-time active learning and model development in pathology. By pre-calculating nucleus-level features, nuclei.io accelerates ML algorithm iteration, enhancing accuracy and transparency in pathologist-AI collaborations. We demonstrated its efficacy in improving prostate cancer and colorectal cancer metastasis detection, highlighting varied benefits across pathologist groups. This framework enhances diagnostic efficiency and prompts further exploration into broader pathology applications, aiming to augment clinical workflows and patient outcomes.
Check out the Paper, Project, and Tool. All credit for this research goes to the researchers of this project. Also, don’t forget to follow us on Twitter.Â
Join our Telegram Channel and LinkedIn Group.
If you like our work, you will love our newsletter..
Don’t Forget to join our 45k+ ML SubReddit
The post Stanford Researchers Launch Nuclei.io: Revolutionizing Artificial Intelligence AI and Clinician Collaboration for Enhanced Pathology Datasets and Models appeared first on MarkTechPost.
Source: Read MoreÂ